Material Transfer Agreements
Material Transfer Agreements help facilitate collaboration while safeguarding proprietary information and ensuring compliance with regulatory requirements.
A Material Transfer Agreement (MTA) is a legal contract that sets the terms for sharing tangible research materials between two organization. These materials can include biological substances like cell lines, plasmids, reagents, as well as chemical compounds and software. MTAs are crucial for protecting the intellectual property (IP) rights of both the provider and the recipient. They specify how the materials can be used, who owns the materials, and any obligations related to the use of the materials.
MTAs:
- specify what materials are being shared and for what research purposes.
- outline the terms and conditions under which the materials can be used
- define the rights of both the provider and the recipient regarding the materials and any derivatives.
No materials should be transferred from or received by Howard University without a fully executed Material Transfer Agreement in place.
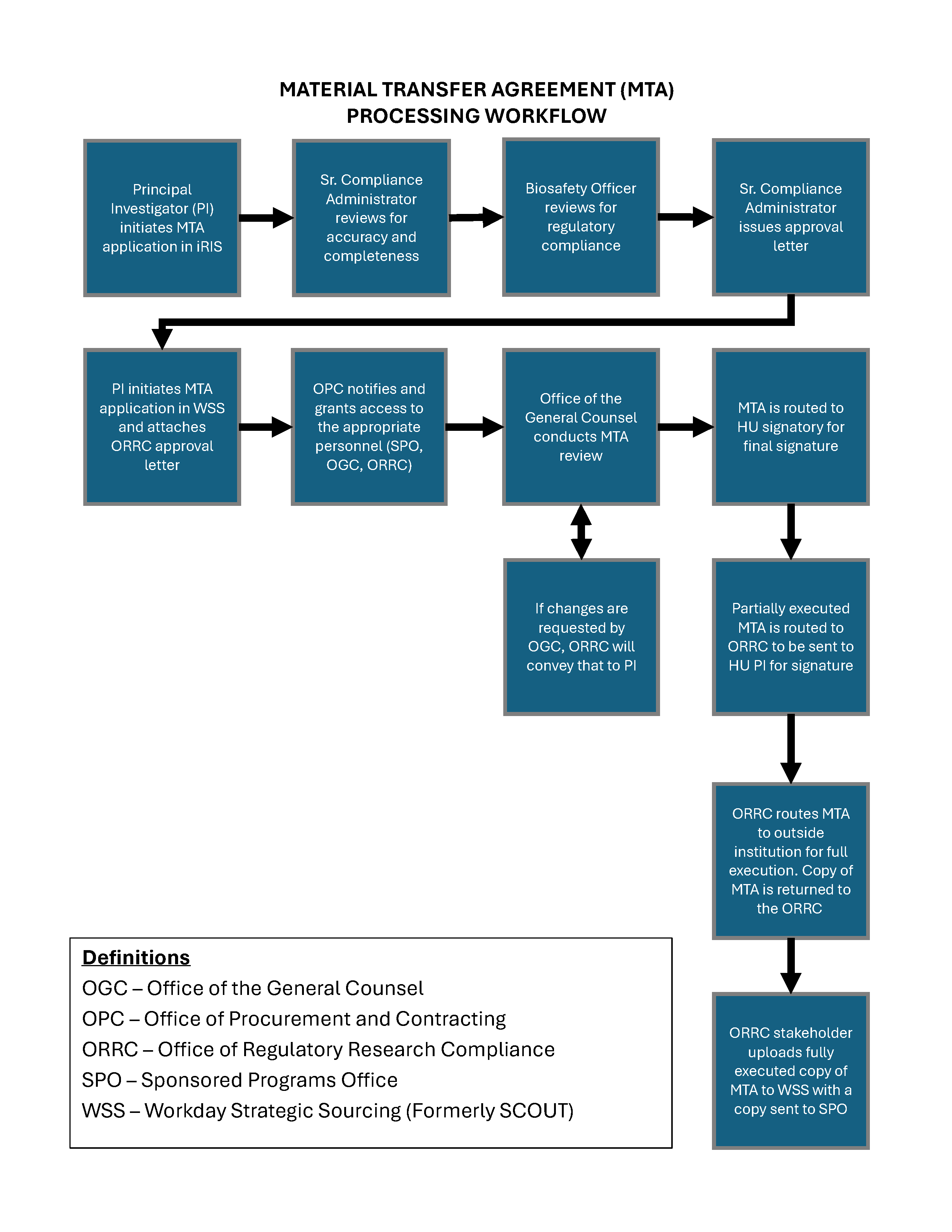
Material Transfer Agreement Approval and Routing Process
Office of Regulatory Research Compliance (ORRC) review through iRIS
- The PI or an authorized study team member creates and completes an MTA Application in the iRiS electronic compliance platform for regulatory compliance review.
-
The ORRC reviews the submission for completeness and accuracy. The ORRC reviews the submission to confirm that the corresponding protocol was approved and for concerns related to Export Control, Research Security, HIPPA, and other regulatory compliance checks. The PI is notified of any necessary corrections and is given the ability to retract and resubmit the Application.
-
The Howard University Biosafety Officer reviews the submission for biosafety concerns. The PI is notified of any necessary corrections and is given the ability to retract and resubmit the Application.
-
If the Application is approved by all parties, an approval letter is issued to the PI from iRIS. This letter must be included in the WSS submission.
Office of Procurement and Contracting (OPC) review through Workday Strategic Sourcing (WSS)
- The PI submits the MTA request in WSS for OPC review. The PI should attach the ORRC MTA Approval letter to this submission.
-
OPC notifies the appropriate personnel and offices of the submission and grants them access to review it. These offices include:
- Office of the General Counsel (OGC) for legal concerns
- Office of Regulatory Research Compliance (ORRC) to verify regulatory compliance
- Sponsored Program Office (SPO) for sponsored program funding concerns
Office of General Counsel (OGC) review through Workday Strategic Sourcing (WSS)
-
The OGC verifies all of the internal approvals for the agreement.
-
The agreement is routed to the following for signature through DocuSign:
- Principal Investigator
- Sponsored Program Office
- External Institution’s authorized signatory
-
The agreement is returned to Howard University.
-
If any revisions are needed, the agreement is routed to the ORRC. If necessary, negotiations between the parties involved (institution, provider, recipient) are conducted to align the terms of the MTA with compliance standards. The revised agreement is routed for signature through DocuSign (Step 2).
-
Upon mutual agreement, the finalized MTA is reviewed and approved by OGC.
-
-
The Office of Procurement and Contracting (OPC) activates the fully executed agreement in WSS. The fully executed agreement is now live.
Material Transfer Agreement Approval and Routing Process
